
Electro-Thermal Bioinstrumentation Laboratory
Jonathan Valvano
Journal Papers and Conference Papers
Areas of expertise within the Electro-Thermal Bioinstrumentation Laboratory include
Embedded systems with mixed analog and digital circuits
Low-power and miniature circuit design including PCB fabrication
Development and integration of real-time operating systems into embedded systems
Low noise data acquisition with real-time digital signal processing
Cosimulation of software, digital signals, analog circuits, and mechanical devices
FEM simulation of electric and thermal fields
Real-time
and Low-power Measurement of Left Ventricular
Volume in Humans using an Admittance Catheter
(with Drs. Feldman and Pearce) sponsored by
Admittance
Technologies
We have developed technology that can be implanted,
providing real-time measurements of end diastolic volume (EDV) and stroke volume
(SV). Continuous monitoring
of left ventricular volume can significantly improve the effectiveness of health
care for patients with chronic heart disease. Measurements of EDV can be used as
an early warning of heart failure. Measurements of SV can be used decide whether
or not to shock a patient during ventricular tachycardia. Measurements of SV
could also be used to select timing parameters of the pacemaker.

In
Vivo Measurement of Left Ventricular
Volume in Laboratory Animals using a Conductance Catheter
(with Drs. Feldman and Pearce) sponsored by
Scisense Inc.
Transgenic
mice offer a valuable method to relate genes to various cardiac diseases, and
pressure-volume analysis is the gold standard for assessing myocardial function.
Cardiac volume can be estimated by a conductance catheter system.
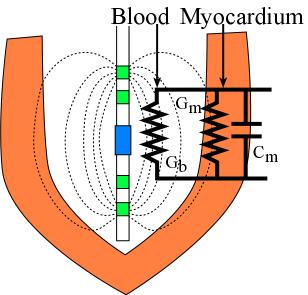
Experimentally a four-electrode catheter is inserted into the mouse left
ventricle (LV) to generate an electric field and continuously to measure the
instantaneous conductance signal. Unfortunately, both blood and myocardium are
conductive, but only the blood conductance is wanted. Therefore, the parallel
myocardium contribution should be removed from the total measured conductance.
Research currently involves FEM numerical studies, instrumentation development,
real time measurement of phase using DSP, in vitro studies and
experimental verification in mice, rats, and humans.
Chronic Measurements of Left Ventricular Volume in Laboratory Animals (with Drs. Feldman and Pearce) sponsored by Admittance Technologies
Transgenic models of heart disease have been created. As a result, it has become
important to accurately and thoroughly evaluate cardiac function in rodents
(mice and rats). Rodent hearts are extremely sensitive to sedation and yield
very different results when conscious studies are compared to those using
anesthesia. Therefore, it is of paramount importance that a complete assessment
of cardiac function is carried out in freely roaming, un-anaesthetized, rodents.
In order to accomplish this goal we must turn to wireless devices that utilize
miniature, lightweight, implants to transmit required data to a nearby base
station. Left ventricular pressure-volume (LV PV) admittance catheter systems
would be ideal for this type of implant, because they are more accurate than
traditional conductance techniques, cheaper than MRI and 2D echocardiography,
and can be implanted in conscious, ambulatory rodents to provide a complete
hemodynamic profile to the academic scientist, and for drug discovery and safety
studies by large pharmaceutical companies. Shown below is a demonstration of
single beat elastance using our wireless admittance system, comparing it to
elastance measured from caval occlusion.
Signal processing in a hearing aid
An audiogram tests the patient's ability to hear sounds across
the audio spectrum. The
sensitivity versus frequency response is used to tune the hearing aid to
compensate for the specific patient's condition. The system involves a number of
creative technologies. A real-time FIR filter shapes the gain versus frequency
response. A nonlinear filter using auto-correlation removes background noise
(shown below).
Time-shifting is a novel approach that moves some high frequency sounds into
lower frequencies, without making it sound like a space alien. The device is
available as a miniature low-power embedded system.
Assessment
of Vulnerable Plaque using Thermal Properties
The overall goal of our project is to develop and evaluate an instrument
that takes a “thermal X-ray” of
the arterial wall. In particular, we propose to
combine sophisticated thermal modeling with precision instrumentation to develop
and evaluate a direct contact probe to scan the arterial wall to detect
vulnerable plaque. The scan will provide the thermal properties of the arterial
wall (namely thermal conductivity). The thermal property measurements will help
us predict the composition of the arterial wall underneath the thermistor-based
sensor. Vulnerable
plaques have more lipid and less fibrous tissue as compared to stable plaques.
There is a strong correlation between the structural components of
biologic tissue (fat, fiber, and calcium) and its thermal properties.
The thermal conductivity of a
material is its ability to transfer heat in the steady state. The proposed
technique is fundamentally different from thermography, which senses an
increased temperature caused by the increased metabolic activity of the plaque.
In contrast, our approach measures thermal conductivity, which in turn
will help to characterize the plaque. In
particular, we believe our instrument will be able to detect the large lipid
core that characterizes vulnerable plaque, and it may also help in determining
the thickness of the fibrous cap.
If
successful, this device will provide a low-cost tool to assess the vulnerability
of plaque, as well as determine the response of the vulnerable plaque to therapy
directed towards improving plaque stability.
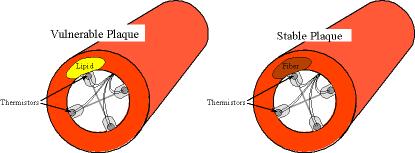
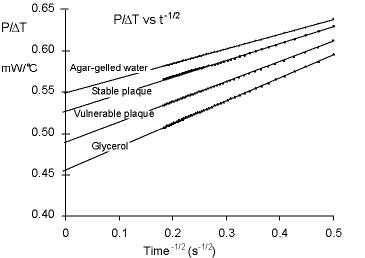
The rationale of our approach is based on the fact that fatty plaques
have a lower thermal conductivity as compared to thermal properties of fibrous
plaques. In particular, lipid has a thermal conductivity that is 60% less than
fibrous tissue. Therefore, we hypothesize that measurements of thermal conductivity will
provide information about the underlying makeup of the plaque, creating a
positive predictor for vulnerable plaque.
The method involves placing a thermal transducer in direct physical
contact with the endothelial surface of the artery under test, delivering a
small burst of heat as well as sensing the tissue temperature response.
The low amount of heat applied for 10-second duration will not have
long-term impacts on the plaque stability or vessel remodeling.
High-Performance A/D Conversion (Robin Tsang,
Byung Geun Lee)
The
overall goal of this project is to develop a high-performance ΔΣ
modulator for analog-to-digital conversion. ΔΣ modulators take
advantage of noise-shaping and oversampling to achieve high resolution .
Noise-shaping is a collective term used to describe feedback systems that use
filtering to push quantization noise out-of-band while leaving in-band signals
unchanged. The main advantage of oversampling is it trades time for dynamic
range. Oversampling also relaxes analog component requirements such as opamp DC
gain and capacitor mismatch tolerances when compared to Nyquist rate converters.
Unfortunately, the overhead of oversampling limits the maximum achievable signal
bandwidth, making ΔΣ modulators attractive only in medium to low speed
applications.
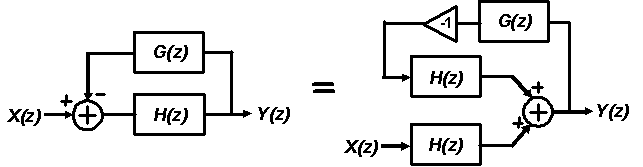
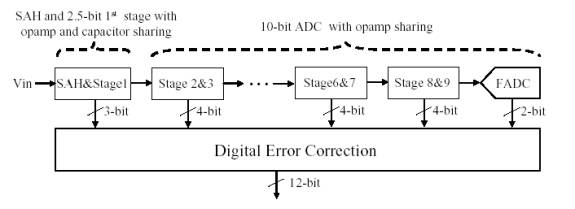
The goal of this project is
to develop a low-power 12-bit 80MS/s pipeline analog-to-digital converter (ADC).
The proposed pipeline ADC
consists of the front-end sample and hold circuit (SAH), 2.5-bit first stage, 8
1.5-bit stages followed by 2-bit flash ADC. In order to reduce power
consumption, the number of op amps will be minimized using an op amp-sharing
technique. In addition, a capacitor sharing technique will be used for the SAH
and the first stage to further reduce power consumption by reducing the SAH
output load. The basic concept of op amp and capacitor sharing technique is
explained in Fig. 2. Since the feedback capacitors of the SAH are directly used
for the first stage MDAC operation, the sampling capacitors of the first stage
are not needed, thus reducing SAH output load capacitance almost by 50%.
Journal Papers and Conference Papers
Go to Home PageLast updated January 21, 2012 Send comments to: Jonathan W. Valvano .